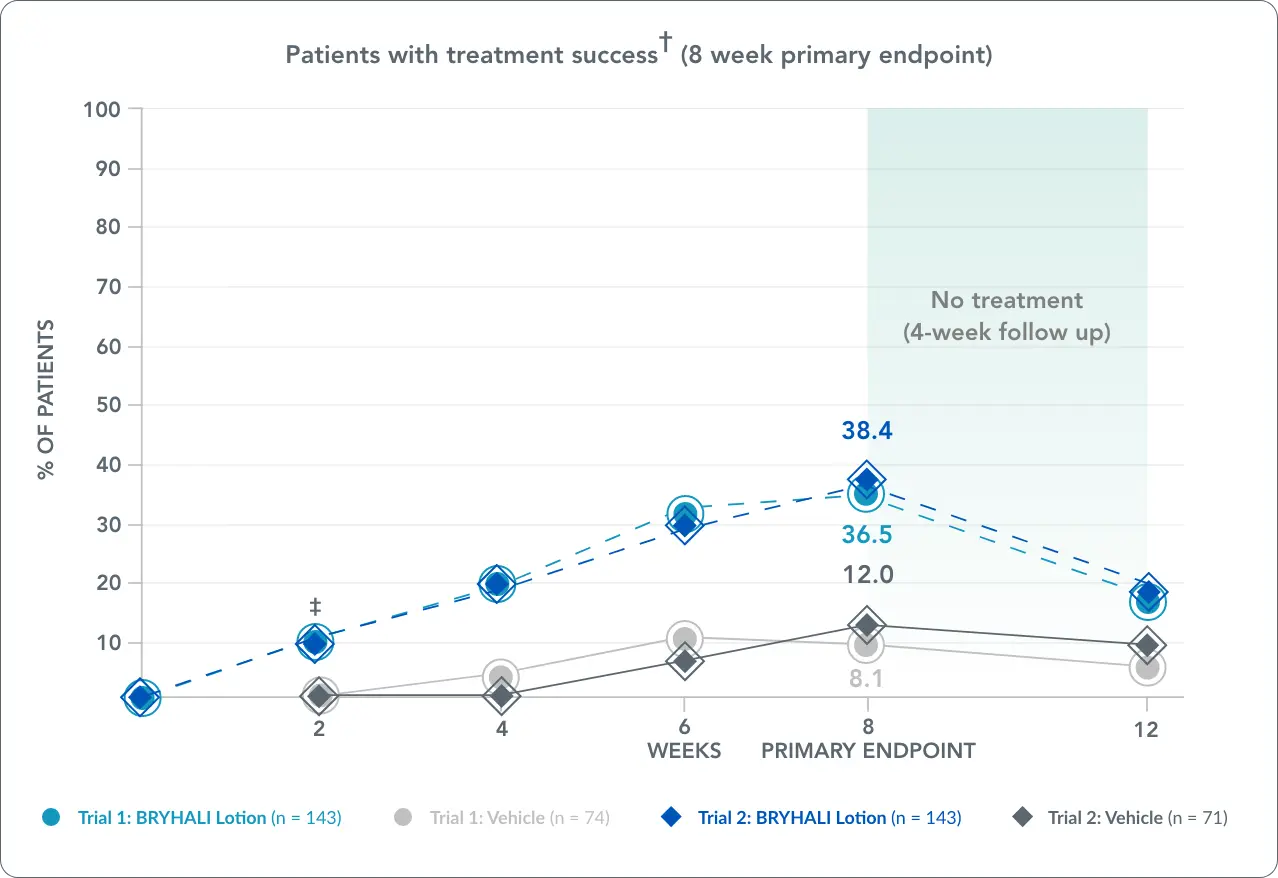
†Treatment success defined as at least a 2-grade improvement from baseline in Investigator's Global Assessment (IGA) score as well as a score of 'clear' or 'almost clear' at week 8 primary endpoint.1,2
‡The treatment difference at week 2 in trial 2 was not statistically significant.1,2
These photos represent actual clinical experience. Photos have not been retouched.
These photos represent actual clinical experience. Photos have not been retouched.
These photos represent actual clinical experience. Photos have not been retouched.
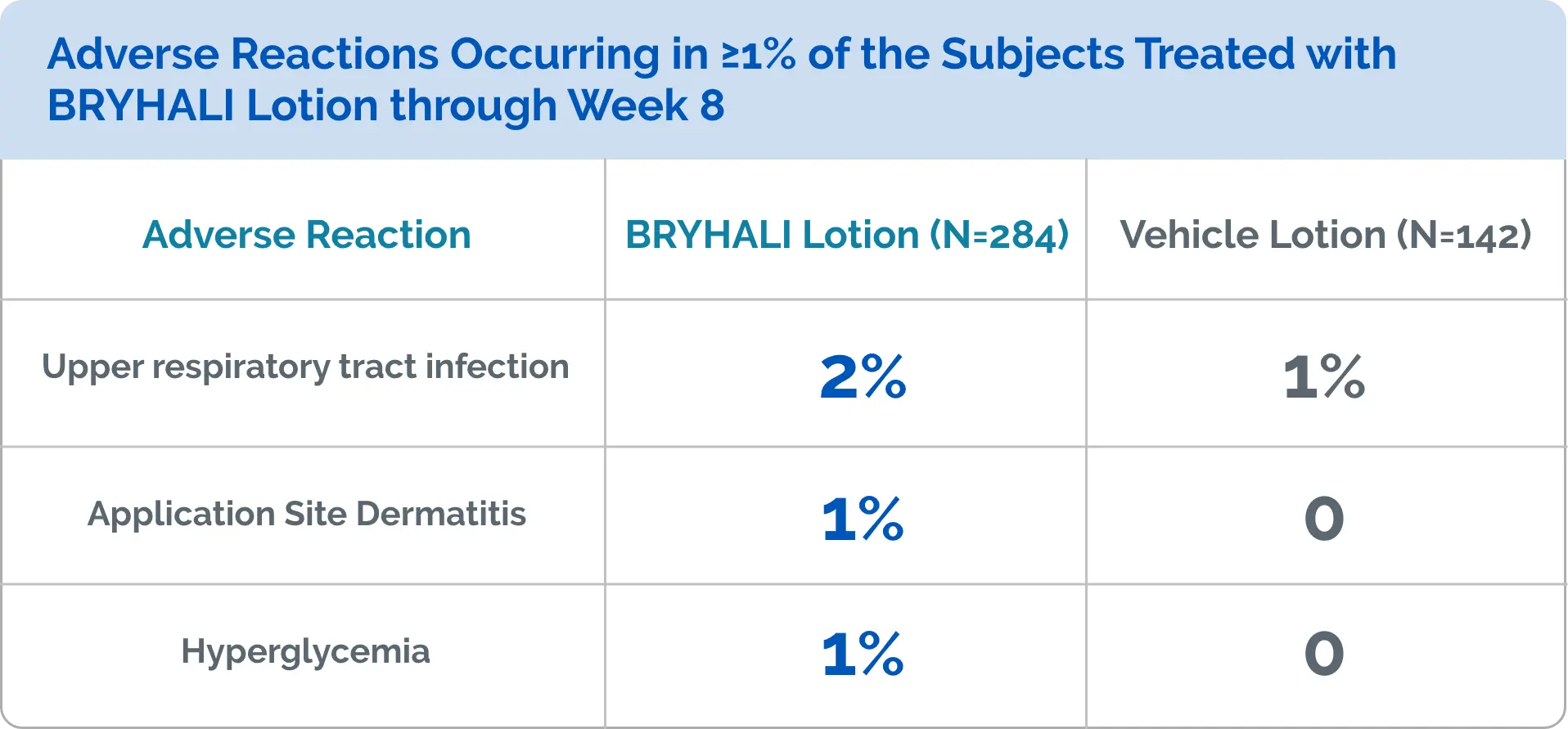
Allergic contact dermatitis with corticosteroids is usually diagnosed by observing failure to heal rather than noting a clinical exacerbation. Consider confirmation of a clinical diagnosis of allergic contact dermatitis by appropriate patch testing. Discontinue BRYHALI Lotion if allergic contact dermatitis occurs.
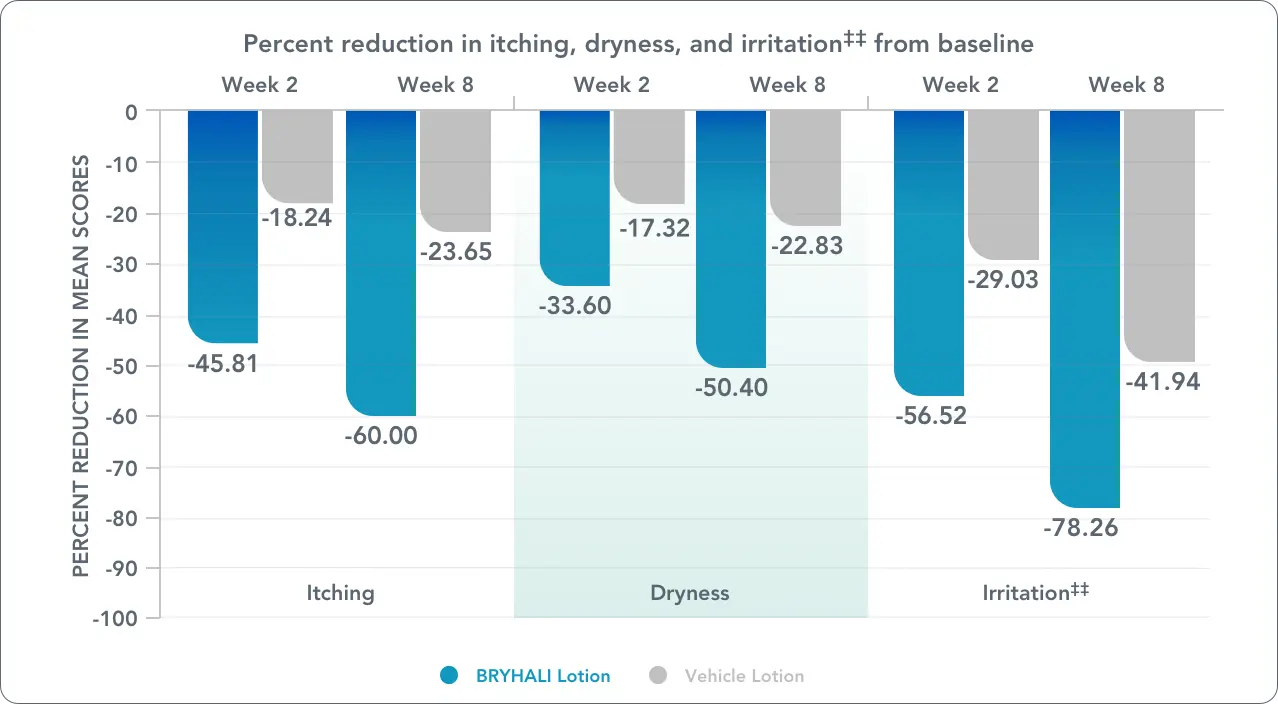
‡‡Reported as burning/stinging in both phase 3 clinical trials.2
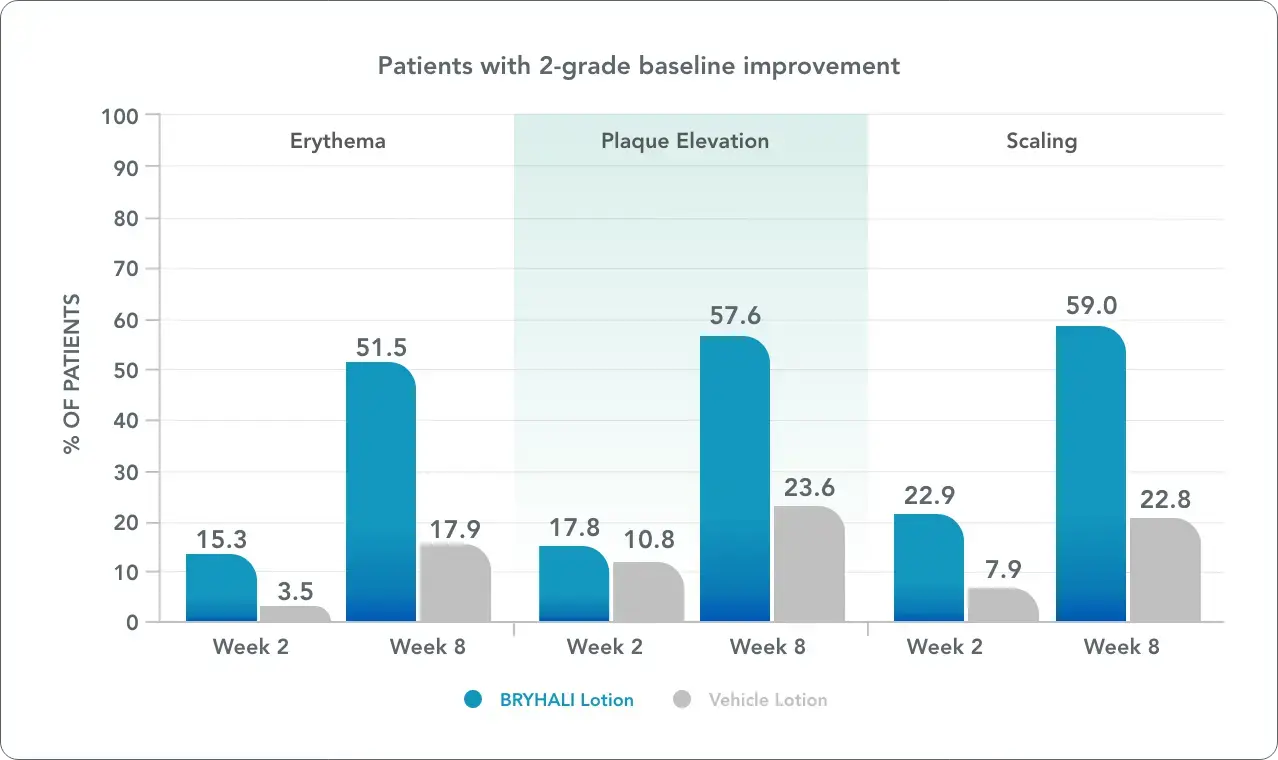
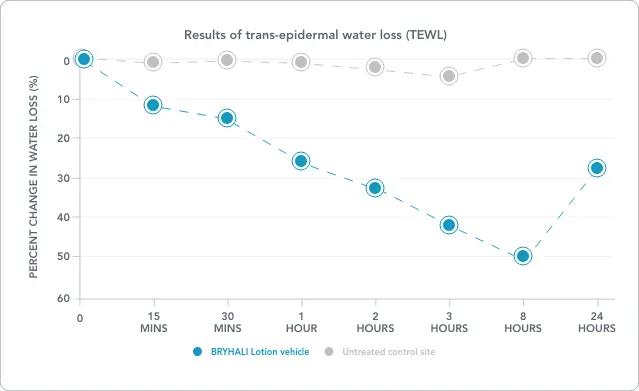
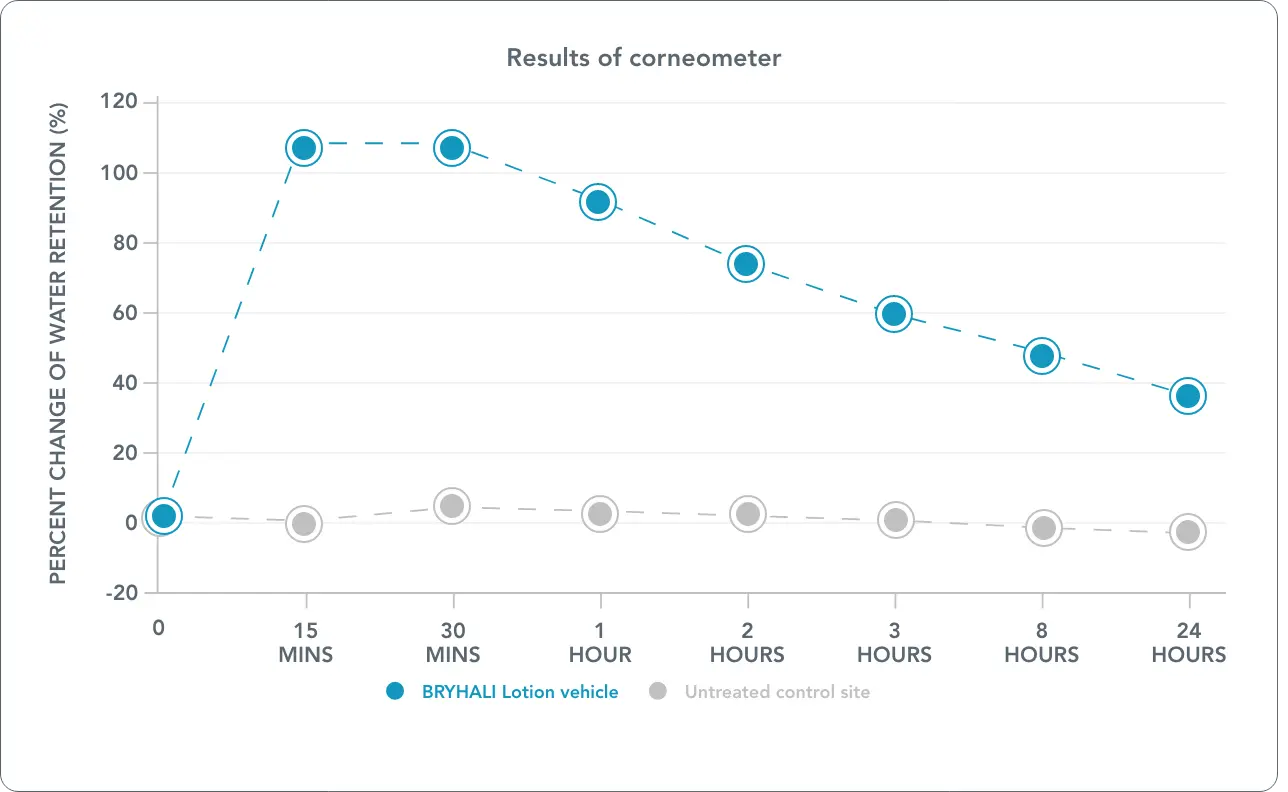
See efficacy data for inflammation, plaque elevation, and scaling
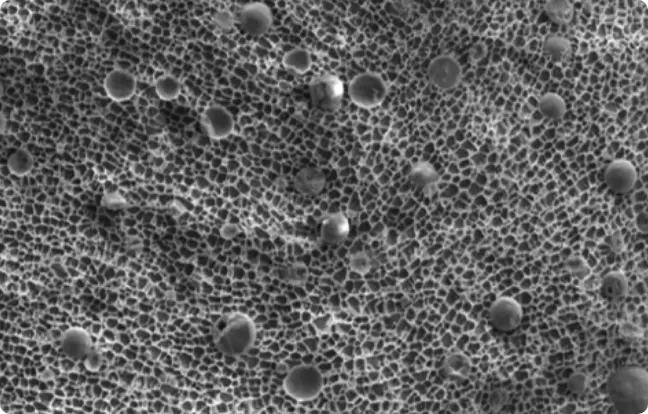
Transmission electron micrograph of BRYHALI Lotion. Magnified 1,000 times.

As little as a $65 co-pay for eligible patients whose commercial insurance does not cover BRYHALI Lotion†
*This offer is only valid for patients with commercial insurance. Uninsured patients are not eligible for this savings offer. This offer is not valid for any person eligible for reimbursement of prescriptions, in whole or in part, by any federal, state, or other governmental programs, including but not limited to Medicare (including Medicare Advantage and Part A, B, and D plans), Medicaid, TRICARE, Veterans Administration, or Department of Defense health coverage, CHAMPUS, the Puerto Rico Government Health Insurance Plan, or any other federal or state health care programs. This offer is good only in the U.S. at retail pharmacies owned and operated by Walgreen Co. (or its affiliates) or other participating independent retail pharmacies. This offer is not valid where otherwise prohibited, taxed, or otherwise restricted. Click here for full eligibility terms and conditions.
†Insured not covered is defined as a patient who has commercial insurance but the drug is not covered on the plan’s formulary or has an NDC block, prior authorization, step edit or other restriction that has not been met.